Reply to the ‘Comment on “Methanol dimer formation drastically enhances hydrogen abstraction from methanol by OH at low temperature”’ by D. Heard, R. Shannon, J. Gomez Martin, R. Caravan, M. Blitz, J. Plane, M. Antiñolo, M. Agundez, E. Jimenez, B. Ballesteros, A. Canosa, G. El Dib, J. Albaladejo and J. Cernicharo, Phys. Chem. Chem. Phys., 2018, 20, DOI: 10.1039/C7CP04561A
Abstract
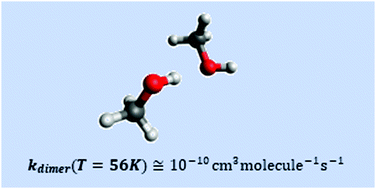
In this Reply we answer the two main arguments raised in the Comment. The first argument is related to the binding energy of the methanol dimer and its influence on the dimerization rate constant. We show that the dimerization rate constants calculated in the Comment are unphysically low. We report values that are about two orders of magnitude higher than the values of the Comment, which confirm the conclusions of the original article that dimers can be present in a small amount. The second argument based on the dependence of the pseudo-first order rates on the methanol concentration was already explained in detail in the Supporting Information of the original article.


 Please wait while we load your content...
Please wait while we load your content...