Direct observation of stepwise intermolecular proton and hydrogen transfer between alcohols and the triplet state of 4-nitro-1-naphthol†
Abstract
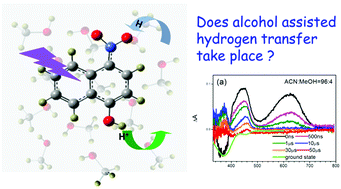
Solvent assisted excited state intramolecular proton or hydrogen transfer has received much attention in bi-functional molecules with hydrogen donating and hydrogen accepting groups. As a typical photoacid, 1-naphthol exhibits photo-stable behavior in methanol; whether this would be disrupted by a bonded hydrogen accepting group contained in the molecule is still not assured. We present nanosecond transient absorption measurements relating to kinetics and the characteristic absorption of key intermediates upon the excitation of 4-nitro-1-naphthol in alcoholic solutions, and also transient resonance Raman spectroscopy studies combined with theoretical calculations to identify the structures of these intermediates, and we reveal the reaction mechanism to be stepwise deprotonation, hydrogen abstraction and protonation. These results demonstrate that alcohol assisted intramolecular proton or hydrogen transfer cannot occur in this system, but that the solvent cluster plays an important role during such stepwise reactions.


 Please wait while we load your content...
Please wait while we load your content...