Molecular dynamics study of the LCST transition in aqueous poly(N-n-propylacrylamide)
Abstract
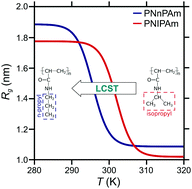
The breadth of technological applications of smart polymers relies on the possibility of tuning their molecular structure to respond to external stimuli. In this context, N-substituted acrylamide-based polymers are widely studied thermoresponsive polymers. Poly(N-n-propylacrylamide) (PNnPAm), which is a structural isomer of the poly(N-isopropylacrylamide) (PNIPAm) exhibits however, a lower phase transition in aqueous solution. In this work, we use all-atom molecular dynamics simulations of PNnPAm in aqueous solutions to study, from a microscopic point-of-view, the influence of chain size and concentration on the LCST of PNnPAm. Our analysis shows that the collapse of a single oligomer of PNnPAm upon heating is dependent on the chain length and corresponds to a complex interplay between hydration and intermolecular interactions. Analysis of systems with multiple chains shows an aggregation of PNnPAm chains above the LCST.


 Please wait while we load your content...
Please wait while we load your content...