Intermolecular interactions between σ- and π-holes of bromopentafluorobenzene and pyridine: computational and experimental investigations
Abstract
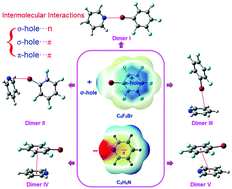
The characters of σ- and π-holes of bromopentafluorobenzene (C6F5Br) enable it to interact with an electron-rich atom or group like pyridine which possesses an electron lone-pair N atom and a π ring. Theoretical studies of intermolecular interactions between C6F5Br and C5H5N have been carried out at the M06-2X/aug-cc-pVDZ level without and with the counterpoise method, together with single point calculations at M06-2X/TZVP, wB97-XD/aug-cc-pVDZ and CCSD(T)/aug-cc-pVDZ levels. The σ- and π-holes of C6F5Br exhibiting positive electrostatic potentials make these sites favorably interact with the N atom and the π ring of C5H5N with negative electrostatic potentials, leading to five different dimers connected by a σ-hole⋯n bond, a σ-hole⋯π bond or a π-hole⋯π bond. Their geometrical structures, characteristics, nature and spectroscopy behaviors were systematically investigated. EDA analyses reveal that the driving forces in these dimers are different. NCI, QTAIM and NBO analyses confirm the existence of intermolecular interactions formed via σ- and π-holes of C6F5Br and the N atom and the π ring of C5H5N. The experimental IR and Raman spectra gave us important information about the formation of molecular complexes between C6F5Br and C5H5N. We expect that the results could provide valuable insights into the investigation of intermolecular interactions involving σ- and π-holes.


 Please wait while we load your content...
Please wait while we load your content...