Characterization of the micelle structure of oleic acid-based gemini surfactants: effect of stereochemistry†
Abstract
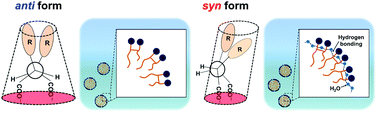
In this study, we synthesize a novel oleic acid-based gemini surfactant with carboxylate headgroups, and study the effect of stereochemistry (anti- vs. syn-) on self-aggregation properties in water. We investigate these properties using phase diagrams, static surface tension, and one-dimensional and two-dimensional nuclear magnetic resonance (NMR) measurements. We find that a phase transition from a hexagonal liquid crystal (H1) phase to a lamellar liquid crystal (Lα) phase occurs at a lower surfactant concentration in the syn form, when compared with the anti form. In addition, the syn form gemini surfactant forms micelles with a close packing of the headgroups via hydrogen bonding. This was supported by static surface tensiometry; the area occupied by surfactant molecules at the air/aqueous solution interface is smaller for the syn form than for the anti form. We propose that, for the syn form gemini surfactant, the closer packing of the headgroups as well as the hydrogen bonding network around the micelle interface prevent water penetration into the hydrophobic part of the micelle.


 Please wait while we load your content...
Please wait while we load your content...