Mechanistic insights into the interaction between energetic oxygen ions and nanosized ZnFe2O4: XAS-XMCD investigations†
Abstract
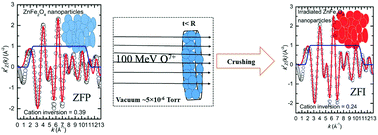
The interactions of energetic ions with multi-cation compounds and their consequences in terms of changes in the local electronic structure, which may facilitate intriguing hybridization between O 2p and metal d orbitals and magnetic ordering, are the subject of debate and require a deep understanding of energy transfer processes and magnetic exchange mechanisms. In this study, nanocrystals of ZnFe2O4 were exposed to O7+ ions with an energy of 100 MeV to understand, qualitatively and quantitatively, the metal–ligand field interactions, cation migration and magnetic exchange interactions by employing X-ray absorption fine structure measurements and X-ray magnetic circular dichroism to get deeper mechanistic insights. Nanosized zinc ferrite nanoparticles (NPs) with a size of ∼16 nm synthesized in the cubic spinel phase exhibited deterioration of the crystalline phase when 100 MeV O7+ ions passed through them. However, the size of these NPs remained almost the same. The behaviour of crystal deterioration is associated with the confinement of heat in this interaction. The energy confined inside the nanoparticles promotes cation redistribution as well as the modification of the local electronic structure. Prior to this interaction, almost 42% of Zn2+ ions occupied AO4 tetrahedra; however, this value increased to 63% after the interaction. An inverse effect was observed for metal ion occupancies in BO6 octahedra. The L-edge spectra of Fe and Zn reveal that the spin and valence states of the metal ions were not affected by this interaction. This effect is also supported by K-edge measurements for Fe and Zn. The t2g/eg intensity ratio in the O K-edge spectra decreased after this interaction, which is associated with detachment of Zn2+ ions from the lattice. The extent of hybridization, as estimated from the ratio of the post-edge to the pre-edge region of the O K-edge spectra, decreased after this interaction. The metal–oxygen and metal–metal bond lengths were modified as a result of this interaction, as determined from extended X-ray absorption fine structure measurements. These measurements further support the observation of cation migration from AO4 tetrahedra to AO6 octahedra and vice versa. The Fe L-edge magnetic circular dichroism spectra indicate that Fe3+ ions occupying sites in AO4 tetrahedra and BO6 octahedra exhibited antiferromagnetic-like ordering prior to this interaction. The NPs that interacted with energetic O ions displayed a different kind of magnetic ordering.


 Please wait while we load your content...
Please wait while we load your content...