Cooperativity between hydrogen- and halogen bonds: the case of selenourea†
Abstract
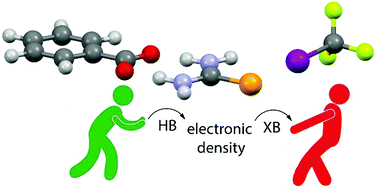
A combined experimental/computational study of cooperativity between halogen-(XB) and hydrogen bonding (HB) is presented. Selenourea (SeU) has been chosen due to its ability to act at the same time as an XB acceptor toward I(CF2)5CF3 (I1) through the two lone pairs on the selenium atom, and as a HB donor to the benzoate anion through its two amino moieties. All equilibrium constants have been estimated using either diffusion NMR and NMR titration techniques. Experimental results demonstrate that the –NH2⋯anion interaction strongly enhances the Se⋯I one of about one order of magnitude (in terms of formation constant of the adduct), whereas DFT results rationalize such results revealing that the presence of a HB between the benzoate and SeU strongly polarizes the latter, enhancing the negative partial charge on selenium and, consequently, its Lewis basicity and its XB acceptor properties.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...