Hydration and ion association of La3+ and Eu3+ salts in aqueous solution†
Abstract
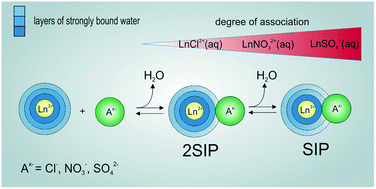
Aqueous solutions of five lanthanide salts: LaCl3, La(NO3)3, La2(SO4)3, Eu(NO3)3 and Eu2(SO4)3 have been studied at 25 °C by dielectric relaxation spectroscopy over the frequency range 0.05 ≤ ν/GHz ≤ 89. Detailed analysis of the solvent-related modes located at higher frequencies showed that both La3+ and Eu3+ are strongly hydrated, even including partial formation of a third hydration shell similar to that of Al3+(aq). Up to two solute-related modes could be detected at lower frequencies, due to the formation of various types of 1 : 1 ion pairs (IPs). All five salts showed modest levels of association in the order Cl− < NO3− ≪ SO42−, mostly in the form of double-solvent-separated IPs with small amounts of solvent-shared IPs. Overall association constants, 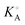 , calculated from the stepwise IP formation constants were consistent with literature values.
, calculated from the stepwise IP formation constants were consistent with literature values.


 Please wait while we load your content...
Please wait while we load your content...