Dissociative adsorption of a multifunctional compound on a semiconductor surface: a theoretical study of the adsorption of hydroxylamine on Ge(100)
Abstract
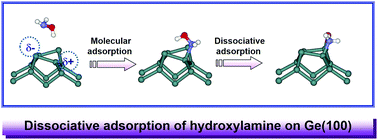
The adsorption behavior of hydroxylamine on a Ge(100) surface was investigated using density functional theory (DFT) calculations. These calculations predicted that hydroxylamine, a multifunctional compound consisting of a hydroxyl group and an amine group, would initially become adsorbed through N-dative bonding, or alternatively through the hydroxyl group via O–H dissociative adsorption. An N–O dissociative reaction may also occur, mainly via N-dative molecular adsorption, and the N–O dissociative product was calculated to be the most stable of all the possible adsorption structures. The calculations furthermore indicated the formation of the N–O dissociative product from the N-dative structure to be nearly barrierless and the dissociated hydroxyl and amine groups to be bonded to two Ge atoms of adjacent Ge dimers. Simulated STM images suggested the change in electron density that would occur upon adsorption of hydroxylamine in various adsorption configurations, and specifically indicated the N–O dissociative product to have greater electron density around the amine groups, and the hydroxyl groups to mainly contribute electron density to the unoccupied electronic states.


 Please wait while we load your content...
Please wait while we load your content...