Photocatalytic selectivity switch to C–C scission: α-methyl ejection of tert-butanol on TiO2(110)†
Abstract
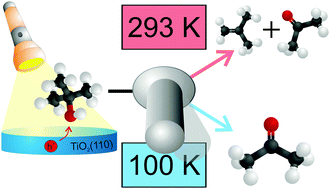
The thermal and photochemical mechanistic pathways for tertiary alcohols on the rutile TiO2(110)-surface are studied with the example of tert-butanol. While the thermal reaction is known to yield isobutene, the photochemical ejection of a methyl radical is observed at 100 K. The C–C scission, which is accompanied by the formation of acetone, is the only photochemical reaction pathway at this temperature and can be attributed to the reaction of photoholes that are created upon UV-light illumination at the surface of the n-type semiconductor. At 293 K the selectivity of the reaction changes, as isobutene is additionally formed photochemically. A comparison of the kinetics of the different reactions reveals further insights. Together with the quantitative evaluation of the reaction products at low temperatures and the comparison of the reaction pathways at different temperatures it is demonstrated how thermal effects can influence the selectivity of the reactions in photocatalysis.


 Please wait while we load your content...
Please wait while we load your content...