XUV photodesorption of carbon cluster ions and ionic photofragments from a mixed methane–water ice
Abstract
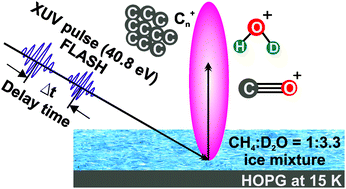
The photochemical processing of a CH4 : D2O 1 : 3.3 ice mixture adsorbed on an HOPG surface in the XUV regime was investigated using pulses obtained from the Free-electron LASer in Hamburg (FLASH) facility. Ice films were exposed to femtosecond pulses with a photon energy of hν = 40.8 eV, consistent with the HeII resonance line. Cationic species desorbing directly from the ice films were detected using time-of-flight (ToF) mass spectrometry. Simple ions formed through the fragmentation of the parent molecules and subsequent recombination reactions were detected and are consistent with efficient D+ and H+ ejection from the parent species, similar to the case for low energy electron irradiation. The FEL fluence dependencies of these ions are linear or exhibit a non-linear order of up to 3. In addition, a series of Cn+ cluster ions (with n up to 12) were also identified. These ions display a highly non-linear desorption yield with respect to the FEL fluence, having an order of 6–10, suggesting a complex multi-step process involving the primary products of CH4 fragmentation. Two-pulse correlation measurements were performed to gain further insight into the underlying reaction dynamics of the photo-chemical reactions. The yield of the D2O derived products displayed a different temporal behaviour with respect to the Cn+ ions, indicating the presence of very different reaction pathways to the two families of ionic products.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...