Synthesis, properties, and formation mechanism of Mn-doped Zn2SiO4 nanowires and associated heterostructures†
Abstract
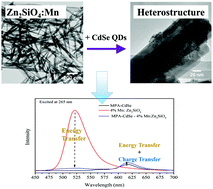
In this work, we have put forth a facile hydrothermal approach to synthesize an array of one-dimensional (1D) Mn-doped Zn2SiO4 nanostructures. Specifically, we have probed and correlated the effects of controllable reaction parameters such as the pH and Mn dopant concentrations with the resulting crystal structures and morphologies of the products obtained. Based upon our results, we find that careful tuning of the pH versus the Mn dopant level gives rise to opposite trends with respect to the overall size of the resulting one-dimensional nanostructures. Significantly, we have highlighted the role of the Mn dopant ion concentration as a potentially generalizable reaction parameter in solution-based synthesis for controlling morphology and hence, the observed optical behavior. Indeed, such a strategy can be potentially generalized to systems such as but not limited to Mn-doped ZnS, CdS, and CdSe quantum dots (QD), which, to the best of our knowledge, denote promising candidates for a variety of optoelectronic applications. Specifically, we have carefully optimized the synthesis conditions in order to generate a series of chemically well-defined Mn-doped Zn2SiO4 not only possessing Mn concentrations ranging from 3% to 8% but also characterized by highly crystalline, monodisperse wire-like motifs measuring ∼30 nm in diameter and ∼700 nm in length. Optically, the photoluminescence signals associated with the 1D series yielded a volcano-shaped relationship between PL intensities and the Mn dopant level. In additional experiments, we have immobilized CdSe quantum dots (QDs) onto the external surfaces of our as-synthesized Mn-doped Zn2SiO4 nanowires, in order to form novel composite heterostructures. The optical properties of the CdSe QD–Mn:Zn2SiO4 heterostructures have been subsequently examined. Our results have demonstrated the likely co-existence of both energy transfer and charge transfer phenomena between the two constituent components of our as-prepared composites. Specifically, when both components are photoexcited, both energy transfer and charge transfer were found to plausibly occur, albeit in opposite directions. When the CdSe QDs are excited alone for example, charge transfer probably takes place from the CdSe QDs to the dopant Mn2+ ions. We believe that our as-processed heterostructures are therefore promising as a tunable light-harvesting motif. Essentially, these materials have broadened the effective light absorption range for optical ‘accessibility’, not only through their incorporation of dopant-tunable Zn2SiO4 possessing complementary absorption properties to those of the QDs but also through their integration of CdSe QDs with size-tailorable optical behavior.


 Please wait while we load your content...
Please wait while we load your content...
