The role of topology in organic molecules: origin and comparison of the radical character in linear and cyclic oligoacenes and related oligomers†
Abstract
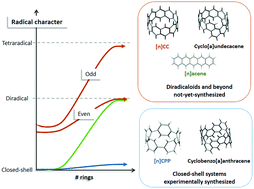
We discuss the nature of electron-correlation effects in carbon nanorings and nanobelts using an analysis tool known as fractional occupation number weighted electron density (ρFOD) and the RAS-SF method, revealing for the first time significant differences in static correlation effects depending on how the rings (i.e. chemical units) are fused and/or connected until closing the loop. We choose to study in detail linear and cyclic oligoacene molecules of increasing size, and relate the emerging differences with the difficulties for the synthesis of the latter due to their radicaloid character. We finally explore how minor structural modifications of the cyclic forms can alter these results, showing the potential use of these systems as molecular templates for the growth of well-shaped carbon nanotubes as well as the usefulness of theoretical tools for molecular design.


 Please wait while we load your content...
Please wait while we load your content...