Exploring the features on the OH + SO2 potential energy surface using theory and testing its accuracy by comparison to experimental data†
Abstract
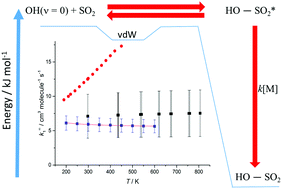
Ab initio theory has been used to identify the pre-reaction complex in the atmospherically important reaction between OH + SO2, (R1), where the binding energy of the pre-reaction complex was determined to be 7.2 kJ mol−1. Using reaction rate theory, implemented with the master equation package MESMER, the effects of this complex on the kinetics of R1 at temperatures above 250 K have been investigated. From simulations and fitting to the experimental kinetic data, it is clear that the influence of this pre-reaction complex is negligible and that the kinetics are controlled by the inner transition-state that leads to the product, HOSO2. While the effect of this complex on the thermal kinetics is small it potentially provides an efficient route to remove energy from vibrationally excited OH. The fitting to the past experimental data reveals that this inner transition-state is submerged with a barrier −0.25 kJ mol−1 below the entrance channel, which is outside the range predicted from the best theoretical calculations. The data fitting also yielded ΔR1H0K equal to −(109 ± 5.6) kJ mol−11 and a more precise expression for k∞1(T), (5.95 ± 0.83) × 10−13 × (T/298)−0.11±0.27.


 Please wait while we load your content...
Please wait while we load your content...