Density functional study on the high catalytic performance of single metal atoms on the NbC(001) surface
Abstract
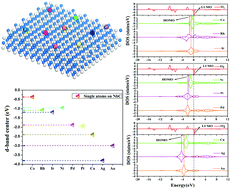
The adsorption and activation of O2 is regarded as the first critical step for the oxygen reduction reaction (ORR), and catalysts with a high performance toward O2 adsorption and activation would provide a theoretical foundation for further investigations. Here, we have studied the adsorption and electronic properties as well as the catalytic activities of group 9–11 single metal atoms deposited on NbC(001), denoted M/NbC(001). According to the location of the d-band centers and the frontier molecular orbital analysis, single metals of Co, Rh, Ir and Ni on NbC(001) exhibit higher activities than other metals (Pd, Pt, Cu, Ag and Au). The quite different catalytic activities of M/NbC(001) may be attributed to the differences in their electro-negativities and work-functions. Meanwhile, the reasonable stabilities of Co, Rh, Ir and Ni on NbC(001) were clarified by investigating the agglomeration resistance and oxidation resistance, and the results indicate that Co and Ni have poor oxidative stability, and Rh and Ir are antioxidants on NbC(001). Further research into the adsorption and activation of O2 confirmed the outstanding properties of Rh/NbC(001) and Ir/NbC(001), which may provide great opportunities to find alternative catalysts.


 Please wait while we load your content...
Please wait while we load your content...