Thermal decomposition of FC(O)OCH3 and FC(O)OCH2CH3†
Abstract
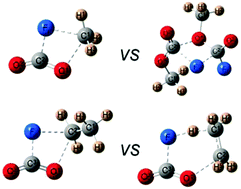
The thermal decomposition of methyl and ethyl formates has been extensively studied due to their importance in the oxidation of several fuels, pesticidal properties and their presence in interstellar space. We hitherto present the study of the thermal decomposition of methyl and ethyl fluoroformates, which could help in the elucidation of the reaction mechanisms. The reaction mechanisms were studied using FTIR spectroscopy in the temperature range of 453–733 K in the presence of different pressures of N2 as bath gas. For FC(O)OCH3 two different channels were observed; the unimolecular decomposition which is favored at higher temperatures and has a rate constant kFC(O)OCH3 = (5.3 ± 0.5) × 1015 exp[−(246 ± 10 kJ mol−1/RT)] (in units of s−1) and a bimolecular channel with a rate constant kFC(O)OCH3 = (1.6 ± 0.5) × 1011 exp[−(148 ± 10 kJ mol−1/RT)] (in units of s−1 (mol L)−1). However for ethyl formate, only direct elimination of CO2, HF and ethylene operates. The rate constants of the homogeneous first-order process fit the Arrhenius equation kFC(O)OCH2CH3 = (2.06 ± 0.09) × 1013 exp[−(169 ± 6 kJ mol−1/RT)] (in units of s−1). The difference between the mechanisms of the two fluoroformates relies on the stabilization of a six-centered transition state that only exists for ethyl formate. First principles calculations for the different channels were carried out to understand the dynamics of the decomposition.


 Please wait while we load your content...
Please wait while we load your content...