Differences in the behavior of dicationic and monocationic ionic liquids as revealed by time resolved-fluorescence, NMR and fluorescence correlation spectroscopy†
Abstract
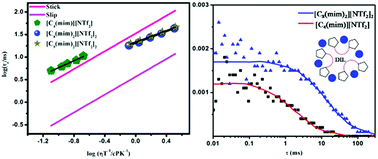
With an aim to understand the behavior in terms of the intermolecular interactions, structure and dynamics of dicationic and monocationic ionic liquids (ILs), two imidazolium-based dicationic ionic liquids (DILs), 1,8-bis-(3-methylimidazolium-1-yl)octane bis-(trifluoromethylsulfonyl)amide ([C8(mim)2][NTf2]2), 1,9-bis-(3-methylimidazolium-1-yl)nonane bis-(trifluoromethylsulfonyl)amide ([C9(mim)2][NTf2]2), and one monocationic ionic liquid (MIL), 1-butyl-3-methyl imidazolium bis(trifluoromethylsulfonyl)amide ([C4(mim)][NTf2]), have been investigated through combined fluorescence, electron paramagnetic resonance (EPR), NMR and fluorescence correlation spectroscopy (FCS). The DILs were synthesized by following a standard synthetic protocol and subsequently characterized by different analytical techniques. Steady state absorption, emission and EPR spectroscopic data reveal that DILs are less polar compared to MIL. The polarities of the DILs and MIL were found to be close to those of acetonitrile and short chain alcohols, respectively. The excitation wavelength dependent emission data reveals that DILs are more micro-heterogeneous in nature than MIL. The rotational diffusion of two organic solutes, perylene and 8-methoxypyrene-1,3,6-sulfonate (MPTS), were examined in the DILs and MIL. The rotational diffusion data for perylene and MPTS were analyzed in light of the Stokes–Einstein–Debye (SED) hydrodynamic theory. The rotation of perylene in the DILs was observed to be relatively faster to that in the MIL, and it goes beyond the limit predicted by the SED theory. In order to explain the rotational motion of perylene in DILs, the data was analyzed further by invoking quasi-hydrodynamic theory. The observed rotational behavior of perylene has been explained by considering the fact that perylene is located in the nonpolar region of ILs, and larger solvent molecules (DILs) induce a lower friction to the rotating solute. Interestingly, unlike perylene, rotations of MPTS in both of the ILs were observed to be much hindered indicating a relatively stronger MPTS–IL interaction than perylene–IL interaction. More interestingly, rotation of MPTS was observed to be faster in the DILs than that in the MIL despite the fact that DILs are more viscous than MILs. Relatively faster rotation of MPTS in DILs has been explained by resorting to NMR and FCS studies. The outcomes of the NMR and FCS studies revealed that DILs in the experimental condition exist in their folded form and because of this structural restriction of DILs it becomes difficult for the bulky MPTS to make stronger hydrogen bonding interactions with DILs, which eventually makes the rotation of MPTS in DILs faster. Essentially, the outcomes of all of these studies have demonstrated that the behavior of DILs is quite different to that of the usual MILs.


 Please wait while we load your content...
Please wait while we load your content...