On the relationship between rutile/anatase ratio and the nature of defect states in sub-100 nm TiO2 nanostructures: experimental insights
Abstract
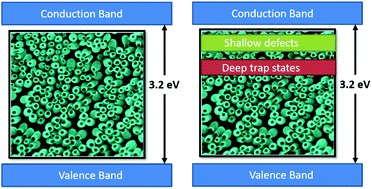
Black TiO2 is being widely investigated due to its superior optical activity and potential applications in photocatalytic hydrogen generation. Herein, the limitations of the hydrogenation process of TiO2 nanostructures are unraveled by exploiting the fundamental tradeoffs affecting the overall efficiency of the water splitting process. To control the nature and concentration of defect states, different reduction rates are applied to sub-100 nm TiO2 nanotubes, chosen primarily for their superiority over their long counterparts. X-Ray Photoelectron Spectroscopy disclosed changes in the stoichiometry of TiO2 with the reduction rate. UV-vis and Raman spectra showed that high reduction rates promote the formation of the rutile phase in TiO2, which is inactive towards water splitting. Furthermore, electrochemical analysis revealed that such high rates induce a higher concentration of localized electronic defect states that hinder the water splitting performance. Finally, incident photon-to-current conversion efficiency (IPCE) highlighted the optimum reduction rate that attains a relatively lower defect concentration as well as lower rutile content, thereby achieving the highest conversion efficiency.


 Please wait while we load your content...
Please wait while we load your content...