Design and properties of functional zwitterions derived from ionic liquids
Abstract
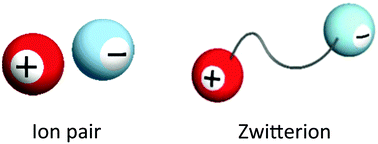
A zwitterion, an ion pair where cation and anion are covalently tethered, is known to be a type of salt. These ions have not been recognised as interesting, but they are physicochemically unique and fascinating ions. In the present review, some functional zwitterions derived from ionic liquids are mentioned to emphasise the usefulness of the tethering of the component cations and anions of ionic liquids. Basic properties, advantages and disadvantages after the functional design of zwitterions, and some applications are summarised.
- This article is part of the themed collection: PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...