Oxidation of substituted aromatic hydrocarbons in the tropospheric aqueous phase: kinetic mechanism development and modelling†
Abstract
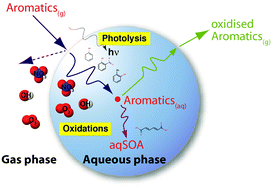
Monocyclic aromatic compounds are ubiquitous in the polluted troposphere and contribute to the formation of tropospheric ozone and anthropogenic secondary organic aerosol, including brown carbon. Currently available physico-chemical data including aqueous-phase kinetic and mechanistic data, as well as phase-transfer parameters have been compiled and reviewed, to construct a novel aqueous-phase oxidation mechanism for monocyclic aromatic compounds. The performed chemical mechanism development results in a comprehensive aqueous-phase oxidation mechanism (addressed as CAPRAM-AM1.0), which includes 292 processes considering the oxidation of different aromatic compounds. Detailed numerical simulations with the air parcel model SPACCIM are carried out for different urban environmental and seasonal conditions. Results show that the aqueous-phase chemistry of aromatic compounds, particularly in clouds, increases the organic aerosol mass by up to 10% in total. The absolute contribution to aqSOA in summertime is modelled to be 260 ng m−3 and 1.2 μg m−3 under moderate and strongly polluted conditions, respectively. Aqueous-phase oxidations of aromatic compounds are important not only for the degradation, but also for the formation of nitrated aromatic compounds. In-cloud chemistry contributes up to 54% to the nitrocatechol oxidation and up to 37% to its formation under polluted tropospheric conditions. Besides, nitrated aromatic compounds contribute up to 5.4 μg m−3 to modelled brown carbon concentration in cloud droplets and 140 ng m−3 in aerosol particles. Further, the model simulations indicate that besides OH radical oxidations, aromatic compounds with two hydroxyl groups are also strongly oxidised by O3 and HO2. O3 contributes with 49% to 68% and HO2 with 19% to 22% to the aqueous-phase oxidation of catechol under moderate and strong polluted environmental conditions studied.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...