Dopant driven tuning of the hydrogen oxidation mechanism at the pore/nickel/zirconia triple phase boundary†
Abstract
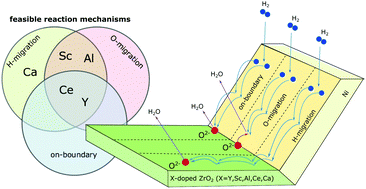
The effects of cation dopants in zirconia on the H2 oxidation mechanism at the pore/nickel/zirconia triple phase boundary (TPB) were theoretically examined. Y, Sc, Al, Ce, and Ca were considered as dopants, and on-boundary, O-migration, and H-migration reaction mechanisms were examined. Based on density functional theory calculations, Y as a dopant favored the on-boundary mechanism with water molecule formation within the immediate proximity of the TPB. The corresponding rate-limiting step is H transfer from the nickel surface to the boundary. In contrast, the on-boundary mechanism is not completed with the Al-, Sc-, and Ca-doped systems, due to the dissociation of water molecules at the boundary. In the Al-doped system, the O-migration mechanism is the major reaction pathway due to a low barrier for the rate-limiting step that corresponds to O transfer from zirconia to the nickel surface. The H-migration mechanism, which implies water molecule formation on the zirconia surface at a position distant from the boundary, should dominate at the Sc-, Ca-, and Ce-doped TPBs, with the lowest activation barrier at the Sc-doped TPB. The reasons for the switching of the reaction mechanisms depending on the dopant species are analyzed.


 Please wait while we load your content...
Please wait while we load your content...
