Origin of the overpotentials for HCOO− and CO formation in the electroreduction of CO2 on Cu(211): the reductive desorption processes decide†
Abstract
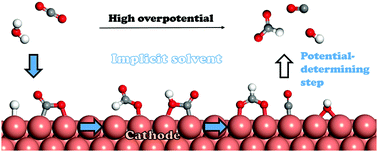
Electroreduction of CO2 on Cu surface provides the potential in producing hydrocarbons and other multi-carbon products. However, a comprehensive understanding of the potential-related mechanism is required to improve the product selectivity as well as to reduce the overpotentials. Herein, we systematically characterize the potential effect on the complete reaction pathways to CO and HCOO− on the Cu(211) surface. Reaction free energy and activation barrier are computed as functions of electric potential. It is found that chemical adsorption state of CO2 is effectively stabilized by the substrate, which is expected to be dominant at potentials below −0.27 V vs. SHE, much earlier than that previously reported on Cu(100). Considering that the activation barriers of the other surface processes are small enough to be overcome at room temperature, the large reductive desorption free energies of OH− and HCOO− are suggested as the origin of high overpotentials.


 Please wait while we load your content...
Please wait while we load your content...