The photolysis of α-hydroperoxycarbonyls†
Abstract
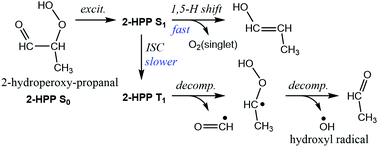
In this work, we theoretically elucidated the mechanism and predicted the major products of the photolysis of α-hydroperoxycarbonyls, known to be products of the atmospheric oxidation of biogenic volatile organic compounds (BVOC) and components of secondary organic aerosol (SOA) in rural and remote areas. Using 2-hydroperoxypropanal OCHCH(OOH)CH3 as a model compound, we show that the likely major photolysis mechanism is a fast 1,5 H-shift in the initially excited singlet S1 state followed by spontaneous elimination of singlet oxygen to yield an enol HOCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, while intersystem crossing (ISC) to the triplet T1 state and C–C scission into HC˙O + HOOC˙HCH3 followed by expulsion of a hydroxyl radical from the unstable HOOC˙HCH3 is another product channel. The direct S1 reaction was found to occur at such a high rate that the quantum yield in atmospheric conditions is expected to approach unity. In the atmosphere, the enol should generally react with OH radicals or tautomerize into the more stable carbonyl O
CHCH3, while intersystem crossing (ISC) to the triplet T1 state and C–C scission into HC˙O + HOOC˙HCH3 followed by expulsion of a hydroxyl radical from the unstable HOOC˙HCH3 is another product channel. The direct S1 reaction was found to occur at such a high rate that the quantum yield in atmospheric conditions is expected to approach unity. In the atmosphere, the enol should generally react with OH radicals or tautomerize into the more stable carbonyl O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2CH3. Vinylalcohol is shown to be a major end product of the photolysis of hydroperoxyacetaldehyde, an isoprene oxidation product. Taking into account also the important enhancement of the absorption cross sections over those of the constituent monofunctional compounds as observed for the analogous β-ketohydroperoxides, (F. Jorand et al., J. Photochem. Photobiol. A: Chem., 2000, 134, 119–125) the atmospheric photolysis rate of α-hydroperoxycarbonyls was estimated to be in the range of (1 to 5) × 10−4 s−1, generally faster than the rate of their OH reactions.
CH–CH2CH3. Vinylalcohol is shown to be a major end product of the photolysis of hydroperoxyacetaldehyde, an isoprene oxidation product. Taking into account also the important enhancement of the absorption cross sections over those of the constituent monofunctional compounds as observed for the analogous β-ketohydroperoxides, (F. Jorand et al., J. Photochem. Photobiol. A: Chem., 2000, 134, 119–125) the atmospheric photolysis rate of α-hydroperoxycarbonyls was estimated to be in the range of (1 to 5) × 10−4 s−1, generally faster than the rate of their OH reactions.


 Please wait while we load your content...
Please wait while we load your content...