Solvation effects on the vibrational modes in hydrated bicarbonate clusters
Abstract
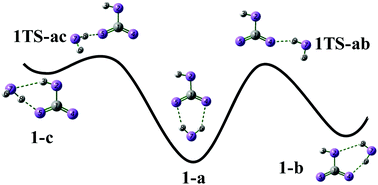
HCO3−(H2O)n clusters provide a model system to understand the solvation interaction between the bicarbonate ion and water. Based on harmonic analysis, ab initio molecular dynamics simulations, and comparison with infrared multiple photon dissociation spectra and with previous results on H2PO4−(H2O)n, the solvation effects on the vibrational modes of HCO3−(H2O)n are analyzed. Hydrogen bond interactions have a significant impact on the vibration, especially when a hydrogen atom is directly involved in a particular mode. The COH bending mode is flattened, when the COH group is solvated by water molecules. The emergence of broad water libration modes indicates the aggregation of water molecules and the formation of a surface structure with bicarbonate on the surface.


 Please wait while we load your content...
Please wait while we load your content...