New carbon allotropes in sp + sp3 bonding networks consisting of C8 cubes
Abstract
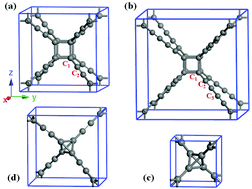
We identify using ab initio calculations new types of three-dimensional carbon allotrope constructed by inserting acetylenic or diacetylenic bonds into a body-centered cubic C8 lattice. The resulting sp + sp3-hybridized cubane-yne and cubane-diyne structures consisting of C8 cubes can be characterized as a cubic crystalline modification of linear carbon chains, but energetically more favorable than the simplest linear carbyne chain and the cubic tetrahedral diamond and yne-diamond consisting of C4 tetrahedrons. Electronic band calculations indicate that these new carbon allotropes are semiconductors with an indirect band gap of 3.08 eV for cubane-yne and 2.53 eV for cubane-diyne. The present results establish new types of carbon phases consisting of C8 cubes and offer insights into their outstanding structural and electronic properties.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...