Magnesium bis(trifluoromethanesulfonyl)amide complexes with triglyme and asymmetric homologues: phase behavior, coordination structures and melting point reduction†
Abstract
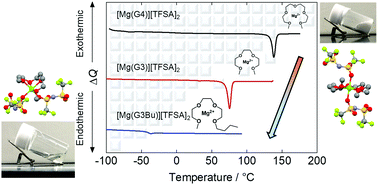
The phase behavior of binary mixtures of triglyme (G3) and Mg[TFSA]2 (TFSA: bis(trifluoromethanesulfonyl)amide) was investigated, towards the development of a Mg2+-based room-temperature solvate ionic liquid (SIL) electrolyte. In a 1 : 1 molar ratio, G3 and Mg[TFSA]2 form a thermally stable complex (decomposition temperature, Td: 240 °C) with a melting point (Tm) of 70 °C, which is considerably lower than that of the analogous tetraglyme (G4) system (137 °C). X-ray crystallography of a single crystal of [Mg(G3)][TFSA]2 revealed that a single Mg2+ cation is coordinated by a single, distorted, tetradentate G3 molecule from one side, and two monodentate [TFSA]− anions, with transoid conformation, from the reverse side to form an ion pair. Raman spectra of [Mg(G3)][TFSA]2 in the molten state revealed the presence of different coordination structures, as the liquid exhibits changes in the vibrational modes corresponding to G3 and the [TFSA]− anion compared to those observed for the solid. Investigation of the ion pair stabilization energies by DFT calculations suggests that higher stability cation complexes and ion pairs co-exist in the molten state than those observed in the crystalline state. These results imply that the coordination structures of the ion pairs play a key role in providing SILs with low Tm. To decrease the Tm further, several asymmetric homologues of G3, which have higher conformational flexibility than G3, were investigated. Notably, a 1 : 1 mixture of Mg[TFSA]2 with G3Bu (where one of the terminal methyl groups of G3 is substituted for a butyl group) formed a thermally stable complex (Td: 251 °C) without any distinct Tm and showed reasonable ionic conductivity at room-temperature, indicating partial dissociation of ions. In this electrolyte, which showed high oxidative stability, quasi-reversible Mg deposition/dissolution was achieved, indicating that Mg2+-based room-temperature SILs can be utilized as a new class of Mg electrolyte.


 Please wait while we load your content...
Please wait while we load your content...