Atomic structure and electronic properties of A2B2XY (A = Si–Pb, B = Cl–I, and XY = PN and SiS) inorganic double helices: first principles calculations†
Abstract
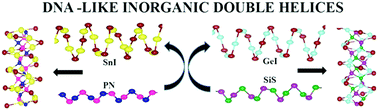
We study the structural stability and electronic properties of new classes of DNA-like inorganic double helices of the type A2B2XY (A = Si–Pb, B = Cl–I, and XY = PN and SiS) by employing first principles density functional theory (DFT) calculations including van der Waals interactions. In these quaternary double helices PN or SiS forms the inner helix while the AB helix wraps around the inner helix and the two are interconnected. We find that the bromides and iodides of Ge, Sn, and Pb as well as Pb2Cl2PN form structurally stable double helices while Ge2I2SiS as well as bromides and iodides of Sn and Pb have stable double helices. The atomic structures of different double helices have been analyzed in detail to understand the stability of these systems as there is up to about 80% difference in the interatomic distances in the two helices which is remarkable. Also in these new classes of double helices there is polar covalent bonding in the inner helix due to heteroatoms. We have calculated the DDEC6 partial atomic charges and bond orders which suggest strong covalent bonding in the inner helix. The electronic structure reveals that these double helices are semiconducting and in many cases the band gap is direct. Furthermore, we have studied the effects of doping and found that hole doping is the most appropriate way to tuning their electronic properties.


 Please wait while we load your content...
Please wait while we load your content...