Self-assemblies of TTF derivatives programmed by alkyl chains and functional groups†
Abstract
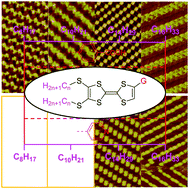
Tetrathiafulvalenes (TTFs) are a class of important functional materials whose intermolecular interaction, which will contribute to constructing a supramolecular structure, still needs further understanding. In this study, the self-assembly behavior and structure of a series of TTFs bearing different alkyl chains and substituents were investigated by scanning tunneling microscopy (STM) in combination with density functional theory (DFT) calculations. Contrary to previous reports, herein, a series of benzoic acid-functionalized TTFs (CnTTFCOOH) and pyridine-functionalized TTFs (CnTTFN) with different lengths of alkyl chains have been substituted on the sulfur atom, where n is equal to 8, 10, 14, or 16. Due to the weak intra- and intermolecular interactions, CnTTFN (n = 8 and 10) molecules cannot be observed during STM scanning. For other cases, various self-assembled monolayers with different nanostructures were observed depending on different substituents. The results reveal that the alkyl chains and functional groups on the TTF skeleton synergistically affect the molecular self-assembly process, which results from the synergism of van der Waals, hydrogen bonding, and S⋯S interactions. These results not only help to explain the relationship between structures and properties, but also help to design better molecular structures for various fields.


 Please wait while we load your content...
Please wait while we load your content...