Hydrogen bond network structures of protonated short-chain alcohol clusters†
Abstract
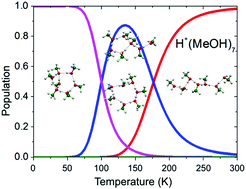
Because of the hydrogen bond coordination properties of alcohols, their possible hydrogen bond network structures are categorized into only a few types. Therefore, gas phase clusters of alcohols can be a very simple model system to examine the properties of hydrogen bond networks, such as structural development with cluster size and temperature dependence. In this perspective, we focus on the structural study of protonated short-chain alcohol clusters, whose excess protons (charge) enable size-selective spectroscopy in combination with mass spectrometric techniques. Size-selective infrared spectroscopy and a theoretical multi-scale isomer search were applied to protonated clusters of methanol, which is a prototype of short-chain alcohols, and their hydrogen bond network development is elucidated in detail. Complete isomer population switching with increasing temperature was predicted by the quantum harmonic superposition approximation and this isomer switching was evidenced by the remarkable temperature (internal vibrational energy) dependence of the observed infrared spectra. The characteristics of the temperature dependence of protonated methanol were compared with those of water and neutral methanol. In addition, possible hydrogen bond networks of methanolated ions were discussed on the basis of the results for protonated methanol. Stepwise changes in the internal energy of clusters with inert gas tagging are demonstrated. Convergence of the hydrogen bond network to the bulk-like network in large clusters is also discussed. The hydrogen bond structures of the protonated clusters of longer normal alkyl chain alcohols (ethanol, 1-propanol, 1-butanol, and 1-pentanol) are determined by comparison of their infrared spectra with those of the protonated methanol clusters. It is demonstrated that the normal alkyl chain interferes only slightly with the most stable hydrogen bond structure, although a few exceptional cases were also found. These exception cases serve as good model systems for further theoretical and computational studies.
- This article is part of the themed collection: PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...