The ortho-benzyne cation is not planar†
Abstract
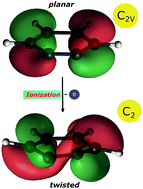
A recent review on the photoionisation of the C6H4 isomer ortho-benzyne suggests that bands reported in earlier photoelectron spectra might be due to side products or contaminations, while computations raise doubts, whether the cation has a planar geometry. We therefore reinvestigate the photoionisation of ortho-benzyne, generated by pyrolysis from benzocyclobutenedione, by photoion mass-selected threshold photoelectron (ms-TPE) spectroscopy using synchrotron radiation. The experiments are accompanied by a theoretical study that investigates the structure of the ortho-benzyne cation systematically as a function of the computational method, up to CASPT2(11,14) ab initio computations. Our study leads to a re-evaluation of the ionisation energy of ortho-benzyne. It reveals that the ortho-benzyne cation has indeed a twisted C2 geometry rather than a C2v structure. A vertical ionisation energy IEvert of 9.77 eV and an adiabatic ionisation energy of IEad = 9.56 eV are computed for ortho-benzyne. A Franck–Condon simulation of the photoelectron spectrum based on the CASPT2 results and including three electronic states of the cation is in agreement with the experiment and yields IEad = 9.51 eV (+50 meV/−100 meV). Since this value is in contrast with previous work, the ionisation energy has to be revised based on our study. Computational methods based on density functional theory give a reasonable description of the cationic ground state, but fail for the corresponding excited electronic states that are indispensible for a proper assignment of the photoelectron spectrum.


 Please wait while we load your content...
Please wait while we load your content...