A catalytic role of surface silanol groups in CO2 capture on the amine-anchored silica support†
Abstract
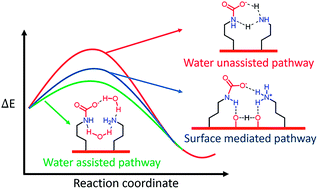
A new mechanism of CO2 capture on the amine-functionalized silica support is demonstrated using density functional theory calculations, in which the silica surface not only acts as a support to anchor amines, but also can actively participate in the CO2 capture process through a facile proton transfer reaction with the amine groups. The surface-mediated proton transfer mechanism in forming a carbamate–ammonium product has lower kinetic barrier (8.1 kcal mol−1) than the generally accepted intermolecular mechanism (12.7 kcal mol−1) under dry conditions, and comparable to that of the water-assisted intermolecular mechanism (6.0 kcal mol−1) under humid conditions. These findings suggest that the CO2 adsorption on the amine-anchored silica surface would mostly occur via the rate-determining proton transfer step that is catalyzed by the surface silanol groups.


 Please wait while we load your content...
Please wait while we load your content...