On the regioselectivity of the Diels–Alder cycloaddition to C60 in high spin states†
Abstract
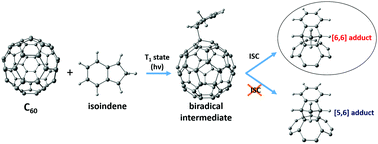
Controlling the regioselectivity in the exohedral functionalization of fullerenes and endohedral metallofullerenes is essential to produce specific desired fullerene derivatives. In this work, using density functional theory (DFT) calculations, we show that the regioselectivity of the Diels–Alder (DA) cycloaddition of cyclopentadiene to 2S+1C60 changes from the usual [6,6] addition in the singlet ground state to the [5,6] attack in high spin states of C60. Changes in the aromaticity of the five- and six-membered rings when going from singlet to high spin C60 provide a rationale to understand this regioselectivity change. Experimentally, however, we find that the DA cycloaddition of isoindene to triplet C60 yields the usual [6,6] adduct. Further DFT calculations and computational analysis give an explanation to this unanticipated experimental result by showing the presence of an intersystem crossing close to the formed triplet biradical intermediate.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...