On the cononsolvency behaviour of hydrophobic clusters in water–methanol solutions
Abstract
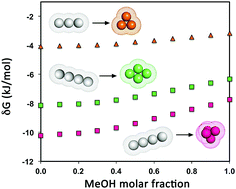
Simple calculations, grounded on the geometric approach to hydrophobic interaction, confirm the occurrence of a minimum in the Gibbs free energy change associated with the formation of several hard sphere clusters in water–methanol solutions with a methanol molar fraction of around 0.3, at room temperature and atmospheric pressure. This finding is in line with the computer simulation results of Mochizuki and Koga [Phys. Chem. Chem. Phys., 2016, 18, 16188]. However, it is underscored that these results cannot be the basis for a rationalization of the cononsolvency phenomenon of the polymers in water–methanol solutions. In fact, there is no Gibbs free energy minimum for the processes more closely resembling polymer collapse, i.e., those involving solely a change in the spatial organization of the same number of hard spheres.


 Please wait while we load your content...
Please wait while we load your content...