Hydrogen bonding and dominant conformations of hydrated sugar analogue complexes using tetrahydrofurfuryl alcohol as the model sugar molecule†
Abstract
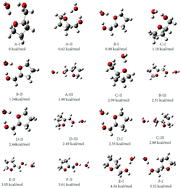
Water molecules, which serve as both hydrogen bond donors and acceptors, have been found to influence the conformational landscape of gas-phase phenyl-β-D-glucopyranoside. Herein, tetrahydrofurfuryl alcohol (THFA), a sugar-like molecule without chromophores (e.g. phenyl-substitution), was used as the model sugar molecule for exploring the behaviour of water molecules on the conformational landscape of a pentose sugar such as deoxyribose. We used mass selected infrared-vacuum ultraviolet (IR-VUV) (118 nm) spectroscopy to investigate the hydrated neutral THFA and its complex cation in a supersonic jet. High level density functional theory (DFT) calculations were performed to ascertain the experimental results. The results revealed that the water molecule tends to insert into the twisted conformer at a position where two stronger intermolecular hydrogen bonds were formed by breaking the weak intramolecular interactions. We found that the twisted conformer of the hydrated neutral THFA complex is more stable than the envelope conformation, while the latter is more stable for the THFA molecule. However, the conformational landscape of the hydrated THFA complex cation did not significantly change on microsolvation with water molecules. These results indicated that the dominant structural landscape of the hydrated cationic complex is the twisted configuration with a trans-hydroxymethyl group. This finding provides valuable insight into the microsolvation of gas-phase sugar molecules.


 Please wait while we load your content...
Please wait while we load your content...