Empirical study of physicochemical and spectral properties of CuII-containing chelate-based ionic liquids†
Abstract
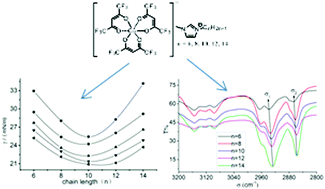
The physicochemical properties including melting point, density, viscosity, conductivity, and surface tension as well as spectral properties such as infrared and EPR spectra of the chelate-based ILs [Cnmim][Cu(F6-acac)3] (n = 6, 8, 10, 12, 14) were studied as functions of temperature and chain length. The thermodynamic properties such as the standard molar entropy and crystal energy were estimated by Glasser's theory, the molar enthalpy of vaporization was calculated by Kabo's method, and the ionicity was estimated by the Walden rule. Compared with the common ILs, the chelate-based ILs have larger molecular volume, larger density, smaller crystal energy, poorer ionicity and larger enthalpy of vaporization. The infrared spectra data of the ILs showed a red shift of the C–H bond stretching vibration of the alkyl chain in the cation and the EPR spectra showed that the crystal field of Cu2+ was kept when the chain length was elongated, which indicated the existence of microphase separation in the ILs. This work is helpful in understanding the structure–property relations of chelate-based ILs for further application.


 Please wait while we load your content...
Please wait while we load your content...