Conformational preferences and isomerization upon excitation/ionization of 2-methoxypyridine and 2-N-methylaminopyridine†
Abstract
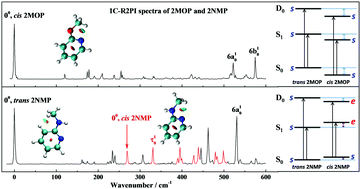
Conformers from the rotations of the methyl group and the methoxy or methylamino group, namely staggered (s)/eclipsed (e)-cis/trans 2-methoxypyridine (2MOP) and 2-N-methylaminopyridine (2NMP), are studied using theoretical calculations in combination with one-color resonance-enhanced two-photon ionization (1C-R2PI) and mass-analyzed threshold ionization (MATI) spectroscopies. The calculations predict that, for cis 2MOP, trans 2MOP and trans 2NMP, only the s conformers are stable in the S0, S1 and D0 states. However, for cis 2NMP, the stable conformer is staggered in the S0 state but eclipsed in the S1 and D0 states, indicating an isomerization upon the excitation or ionization from the S0 state. This is experimentally supported by the 1C-R2PI and MATI spectra of 2NMP. Due to the relative instability, the number density of trans 2MOP is too low in the sample to be detected. All the bands in the 1C-R2PI and MATI spectra of 2MOP are assigned to s-cis 2MOP. The energy differences between cis and trans conformers are derived from excitation and ionization energies, indicating another conformational isomerization: stable trans 2NMP in the S0 and S1 states but stable cis 2NMP in the D0 state. For 2MOP, the so-called syn preference previously found for the S0 state is also observed in the S1 and D0 states. The conformational preference and isomerization are discussed with natural bond orbital calculations and reduced density gradient analysis. For 2MOP, the syn preferences are mainly caused by the exchange repulsion among several σ-orbitals of the OCH3 group and the pyridine ring. While the relative stabilities of the s and e conformers of cis 2MOP and cis 2NMP are simultaneously influenced by steric repulsion and orbital interactions.


 Please wait while we load your content...
Please wait while we load your content...