Kinetics of the reaction of CO3˙−(H2O)n, n = 0, 1, 2, with nitric acid, a key reaction in tropospheric negative ion chemistry†
Abstract
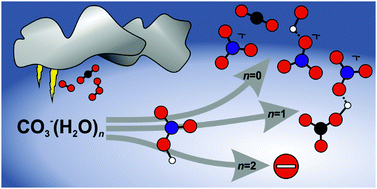
A significant fraction of nitrate in the troposphere is formed in the reactions of HNO3 with the carbonate radical anion CO3˙− and the mono- and dihydrated species CO3˙−(H2O)1,2. A reaction mechanism was proposed in earlier flow reactor studies, which is investigated here in more detail by quantum chemical calculations and experimental reactivity studies of mass selected ions under ultra-high vacuum conditions. Bare CO3˙− forms NO3−(OH˙) as well as NO3−, with a total rate coefficient of 1.0 × 10−10 cm3 s−1. CO3˙−(H2O) in addition affords stabilization of the NO3−(HCO3˙) collision complex, and thermalized CO3˙−(H2O) reacts with a total rate coefficient of 6.3 × 10−10 cm3 s−1. A second solvent molecule quenches the reaction, and only black-body radiation induced dissociation is observed for CO3˙−(H2O)2, with an upper limit of 6.0 × 10−11 cm3 s−1 for any potential bimolecular reaction channel. The rate coefficients obtained under ultra-high vacuum conditions are smaller than in the earlier flow reactor studies, due to the absence of stabilizing collisions, which also has a strong effect on the product branching ratio. Quantum chemical calculations corroborate the mechanism proposed by Möhler and Arnold. The reaction proceeds through a proton-transferred NO3−(HCO3˙) collision complex, which can rearrange to NO3−(OH˙)(CO2). The weakly bound CO2 easily evaporates, followed by evaporation of the more strongly attached OH˙, if sufficient energy is available.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...