Electrochemical behavior of Bi4B2O9 towards lithium-reversible conversion reactions without nanosizing†
Abstract
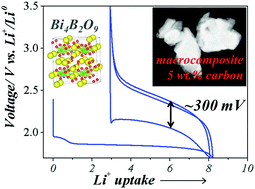
Conversion type materials, in particular metal fluorides, have emerged as attractive candidates for positive electrodes in next generation Li-ion batteries (LIBs). However, their practical use is being hindered by issues related to reversibility and large polarization. To minimize these issues, a few approaches enlisting the anionic network have been considered. We herein report the electrochemical properties of bismuth oxyborate Bi4B2O9 and show that this compound reacts with lithium via a conversion reaction leading to a sustained capacity of 140 mA h g−1 when cycled between 1.7 and 3.5 V vs. Li+/Li0 while having a surprisingly small polarization (∼300 mV) in the presence of solely 5% in weight of a carbon additive. These observations are rationalized in terms of charge transfer kinetics via complementary XRD, HRTEM and NMR measurements. This finding demonstrates that borate based conversion type materials display rapid charge transfer with limited carbon additives, hence offering a new strategy to improve their overall cycling efficiency.


 Please wait while we load your content...
Please wait while we load your content...