Nanoemulsions stabilized by non-ionic surfactants: stability and degradation mechanisms
Abstract
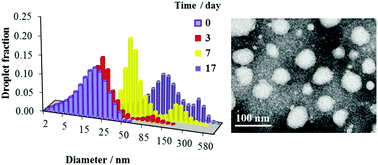
The prevailing opinion in the literature is that the main mechanism of O/W nanoemulsion degradation is Ostwald ripening. Nevertheless, the experimental rates of Ostwald ripening are usually several orders of magnitude higher than the theoretical values. This suggests that other mechanisms, such as coalescence, flocculation and subsequent creaming, significantly influence nanoemulsion breakdown. We investigated O/W nanoemulsions stabilized by Brij 30 or by a mixture of Tween 80 and Span 80 and with liquid paraffin as a dispersed phase. The results indicate that Ostwald ripening is the main process leading to nanoemulsion coarsening only in nanoemulsions with low oil phase fractions of up to 0.05. For quasi-steady state conditions the rates of Ostwald ripening are equal to (1.5 ± 0.3) × 10−29 and (1.1 ± 0.3) × 10−29 m3 s−1 in nanoemulsions with Brij 30 and Tween 80 & Span 80, respectively. In nanoemulsions with oil phase fractions of 0.15–0.45, different mechanisms are identified. Flocculation prevails over other processes during the first days in nanoemulsions stabilized by Brij 30. Coalescence is the main mechanism of nanoemulsion degradation for long times. An increase in droplet size 5–10 days after nanoemulsion preparation due to Ostwald ripening takes place in the case of nanoemulsion stabilization by Tween 80 and Span 80. The stability behavior of these nanoemulsions at later stages is distinctly affected by coalescence and flocculation.


 Please wait while we load your content...
Please wait while we load your content...