Full-dimensional analytical potential energy surface describing the gas-phase Cl + C2H6 reaction and kinetics study of rate constants and kinetic isotope effects†
Abstract
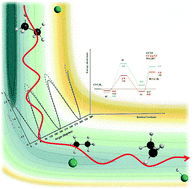
Within the Born–Oppenheimer approximation a full-dimensional analytical potential energy surface, PES-2017, was developed for the gas-phase hydrogen abstraction reaction between the chlorine atom and ethane, which is a nine body system. This surface presents a valence-bond/molecular mechanics functional form dependent on 60 parameters and is fitted to high-level ab initio calculations. This reaction presents little exothermicity, −2.30 kcal mol−1, with a low height barrier, 2.44 kcal mol−1, and intermediate complexes in the entrance and exit channels. We found that the energetic description was strongly dependent on the ab initio level used and it presented a very flat topology in the entrance channel, which represents a theoretical challenge in the fitting process. In general, PES-2017 reproduces the ab initio information used as input, which is merely a test of self-consistency. As a first test of the quality of the PES-2017, a theoretical kinetics study was performed in the temperature range 200–1400 K using two approaches, i.e. the variational transition-state theory and quasi-classical trajectory calculations, with spin–orbit effects. The rate constants show reasonable agreement with experiments in the whole temperature range, with the largest differences at the lowest temperatures, and this behaviour agrees with previous theoretical studies, thus indicating the inherent difficulties in the theoretical simulation of the kinetics of the title reaction. Different sources of error were analysed, such as the limitations of the PES and theoretical methods, recrossing effects, and the tunnelling effect, which is negligible in this reaction, and the manner in which the spin–orbit effects were included in this non-relativistic study. We found that the variation of spin–orbit coupling along the reaction path, and the influence of the reactivity of the excited Cl(2P1/2) state, have relative importance, but do not explain the whole discrepancy. Finally, the activation energy and the kinetics isotope effects reproduce the experimental information.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...