Importance of protein flexibility on molecular recognition: modeling binding mechanisms of aminopyrazine inhibitors to Nek2
Abstract
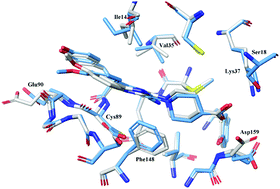
NIMA-related kinase 2 (Nek2) plays a significant role in cell cycle regulation, and overexpression of Nek2 has been observed in several types of carcinoma, suggesting it is a potential target for cancer therapy. In this study, we attempted to gain more insight into the binding mechanisms of a series of aminopyrazine inhibitors of Nek2 through multiple molecular modeling techniques, including molecular docking, molecular dynamics (MD) simulations and free energy calculations. The simulation results showed that the induced fit docking and ensemble docking based on multiple protein structures yield better predictions than conventional rigid receptor docking, highlighting the importance of incorporating receptor flexibility into the accurate predictions of the binding poses and binding affinities of Nek2 inhibitors. Additionally, we observed that the Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) calculations did not show better performance than the docking scoring to rank the binding affinities of the studied inhibitors, suggesting that MM/GBSA is system-dependent and may not be the best choice for the Nek2 systems. Moreover, the detailed information on protein–ligand binding was characterized by the MM/GBSA free energy decomposition, and a number of derivatives with improved docking scores were designed. It is expected that our study can provide valuable information for the future rational design of novel and potent inhibitors of Nek2.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...