Can electrostatic catalysis of Diels–Alder reactions be harnessed with pH-switchable charged functional groups?†
Abstract
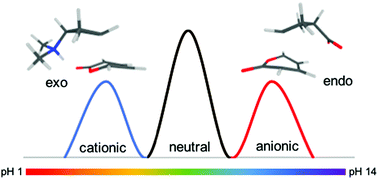
Quantum-chemical calculations at the M06-2X/6-31+G(d,p) and G3(MP2)CC levels of theory are used to assess the feasibility of harnessing charged functional groups to electrostatically catalyse Diels–Alder reactions and alter their regio selectivity. For the reaction of the polar diene 2-pyrone with substituted cyclopentene, pH switches of nearly 60 kJ mol−1 are observed in the gas-phase. To switch regioselectivity however it is necessary to toggle between negatively and positively charged functional groups. With the 6-membered cyclohexene derivatives, similar pH-switches are observed but this time an opportunity to pH-switch diastereomeric selectivity is also observed due to the asymmetry of the transition state. When 2-pyrone was replaced with a non-polar diene, cyclopentadiene, pH switches were understandably smaller but still substantial (ca. 15 kJ mol−1). Likewise pH switches are attenuated by solvent but remain substantial (ca. 30 kJ mol−1) in toluene and synthetically useful (ca. 15 kJ mol−1) even in moderately low polar solvents such as dichloromethane.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...