Ab initio calculations and kinetic modeling of thermal conversion of methyl chloride: implications for gasification of biomass†
Abstract
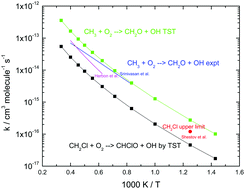
Limitations in current hot gas cleaning methods for chlorine species from biomass gasification may be a challenge for end use such as gas turbines, engines, and fuel cells, all requiring very low levels of chlorine. During devolatilization of biomass, chlorine is released partly as methyl chloride. In the present work, the thermal conversion of CH3Cl under gasification conditions was investigated. A detailed chemical kinetic model for pyrolysis and oxidation of methyl chloride was developed and validated against selected experimental data from the literature. Key reactions of CH2Cl with O2 and C2H4 for which data are scarce were studied by ab initio methods. The model was used to analyze the fate of methyl chloride in gasification processes. The results indicate that CH3Cl emissions will be negligible for most gasification technologies, but could be a concern for fluidized bed gasifiers, in particular in low-temperature gasification. The present work illustrates how ab initio theory and chemical kinetic modeling can help to resolve emission issues for thermal processes in industrial scale.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...