The mechanism of electrochemical reduction of hydrogen peroxide on silver nanoparticles†
Abstract
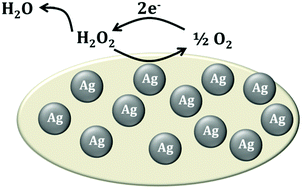
The reduction of hydrogen peroxide on a silver nanoparticle modified boron doped diamond electrode in a neutral solution is shown to proceed through a CE mechanism. Hydrogen peroxide undergoes a disproportionation reaction to form oxygen (and water) on the silver surface, creating a diffusion layer of oxygen, which, at a sufficiently biased electrode, is then reduced to hydrogen peroxide. Voltammetry and a full mechanistic simulation are undertaken to confirm the mechanism, showing at short times a dependence of the reductive signal on waiting time prior to voltammetric analysis reflecting the extent of the disproportionation step which occurs prior to voltammetric analysis.


 Please wait while we load your content...
Please wait while we load your content...