Chemical tracer diffusion of Sr and Co in polycrystalline Ca-deficient CaMnO3−δ with CaMn2O4 precipitates
Abstract
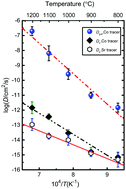
Diffusivity on the A- and B-site of polycrystalline perovskite CaMnO3−δ with Ca deficiency and spinel CaMn2O4 (marokite) as a secondary phase was studied using chemical tracers and secondary ion mass spectrometry (SIMS) complemented by electron probe microanalysis (EPMA). Thin films containing Sr and Co chemical tracers were deposited on the polished surface of the polycrystalline composite sample followed by annealing at 800–1200 °C for 96 h. Diffusion profiles for each tracer were determined with SIMS, followed by calculation of diffusion coefficients by fitting to appropriate models. The Sr tracer showed mainly lattice diffusion, with an activation energy of 210 ± 30 kJ mol−1, whereas the Co tracer showed a combination of lattice and enhanced grain-boundary diffusion, with activation energies of 270 ± 30 kJ mol−1 and 380 ± 40 kJ mol−1, respectively. The diffusivities may be used to predict interdiffusion and lifetime of junctions between n-type CaMnO3−δ or CaMnO3−δ/CaMn2O4 composites and metallization interlayers or p-type leg materials in oxide thermoelectrics. In particular, the relatively high effective diffusivity of Co in polycrystalline CaMnO3−δ may play a role in the reported fast formation of the secondary phase (Ca3Co2−yMnyO6) between p-type Ca3Co3.92O9+δ and n-type CaMnO3−δ in a direct p–n thermoelectric junction.


 Please wait while we load your content...
Please wait while we load your content...