Effect of heteroatom and functionality substitution on the oxidation potential of cyclic nitroxide radicals: role of electrostatics in electrochemistry†
Abstract
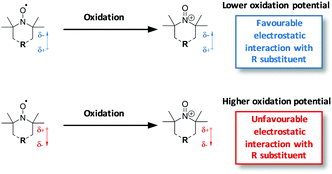
The oxidation potential of a test set of 21 nitroxide radicals, including a number of novel compounds, has been studied experimentally in acetonitrile and correlated with theoretical calculations. It was found that both Hammett constants (σp) of the substituents on the nitroxide radicals and hyperfine splitting constants of the respective nitrogen atoms (αN) were well correlated to their experimental oxidation potentials. Theoretical calculations, carried out at the G3(MP2,CC)(+)//M06-2X/6-31+G(d,p) level of theory with PCM solvation corrections, were shown to reproduce experiments to within a mean absolute deviation of 33 mV, with a maximum deviation of 64 mV. The oxidation potentials of the nitroxides examined varied over 400 mV, depending on ring size and substitution. This considerable variation can be rationalised by the ability of various substituents to electrostatically stabilize the oxidised oxoammonium cation. Importantly, this can be quantified by a simple predictive relationship involving the distance scaled dipole and quadrupole moments of the analogous cyclohexyl ring. This highlights the often-overlooked role of through-space electrostatic substituent effects, even in formally neutral compounds.


 Please wait while we load your content...
Please wait while we load your content...