Effect of osmolytes on the thermal stability of proteins: replica exchange simulations of Trp-cage in urea and betaine solutions†
Abstract
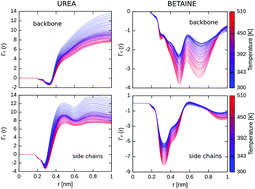
Although osmolytes are known to modulate the folding equilibrium, the molecular mechanism of their effect on thermal denaturation of proteins is still poorly understood. Here, we simulated the thermal denaturation of a small model protein (Trp-cage) in the presence of denaturing (urea) and stabilizing (betaine) osmolytes, using the all-atom replica exchange molecular dynamics simulations. We found that urea destabilizes Trp-cage by enthalpically-driven association with the protein, acting synergistically with temperature to induce unfolding. In contrast, betaine is sterically excluded from the protein surface thereby exerting entropic depletion forces that contribute to the stabilization of the native state. In fact, we find that while at low temperatures betaine slightly increases the folding free energy of Trp-cage by promoting another near-native conformation, it protects the protein against temperature-induced denaturation. This, in turn, can be attributed to enhanced exclusion of betaine at higher temperatures that arises from less attractive interactions with the protein surface.


 Please wait while we load your content...
Please wait while we load your content...