Transition of surface phase of cobalt oxide during CO oxidation
Abstract
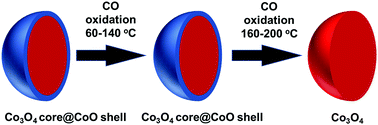
In situ/operando studies of a heterogeneous catalyst are particularly valuable for achieving a fundamental understanding of catalytic mechanisms at a molecular level by establishing a correlation between the observed catalytic performance and the corresponding surface chemistry during catalysis. Herein, CO oxidation on cobalt oxides was studied via ambient pressure X-ray photoelectron spectroscopy (AP-XPS). During CO oxidation on CoO in the temperature range of 140–180 °C, the active surface phase of CoO progressively transforms to Co3O4. Kinetic studies of CO oxidation on the surface phase CoO at 80–120 °C and on the formed Co3O4 at 160–220 °C show that CoO and Co3O4 exhibit different activation barriers: 49.3 kJ mol−1 for CoO and 36.9 kJ mol−1 for Co3O4. This study demonstrates the transition of the active surface phase of a transition metal oxide-based catalyst under catalytic conditions with no change in the bulk phase of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...