Optical absorption and photoconductivity in iodine-excess ionic liquids: the case of 1-alkyl-3-methyl imidazolium iodides
Abstract
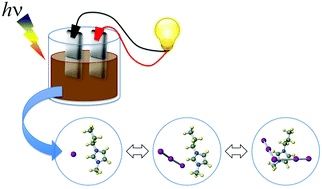
We investigated the optical absorption and photoconductivity of iodine-excess ionic liquids (ILs) based on 1-alkyl-3-methyl imidazolium iodide ([Cnmim][I]; n = 3, 4, and 6). The iodide concentration m was 2 ≦ m ≦ 8, which was determined by the molar fraction [Cnmim]+ : [Im]− = 1 : m. By adding iodine, an absorption edge shifted from 282 nm in the UV region to around 600 nm in the visible-light region. The optical bandgaps Eo decreased gradually from 2.3 eV to 1.9 eV with increasing m from 2 to 8. The alkyl-side chain lengths of the cations have little effect on the Eo. This experimental result was confirmed by ab initio molecular orbital calculations. The effects were reflected in the photoconductivity of the ILs, as expected. [C4mim][Im] exhibited greater photo-induced electron generation compared with [C3mim][Im] and [C6mim][Im]. The photoconductivity in both [C3mim][Im] and [C6mim][Im] increased slightly with increasing m. The trend of photoconductivity in [C4mim][Im] exhibited an N-shaped form. The highest photoconductivity 1.6 was observed in [C4mim][I8].


 Please wait while we load your content...
Please wait while we load your content...