Atmospheric chemistry of CH3CHO: the hydrolysis of CH3CHO catalyzed by H2SO4†
Abstract
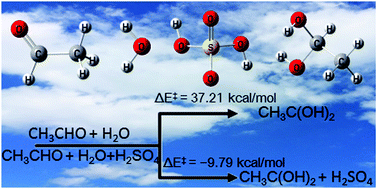
Elucidating atmospheric oxidation mechanisms and the reaction kinetics of atmospheric compounds is of great importance and necessary for atmospheric modeling and the understanding of the formation of atmospheric organic aerosols. While the hydrolysis of aldehydes has been detected in the presence of sulfuric acid, the reaction mechanism and kinetics remain unclear. Herein, we use electronic structure methods with CCSD(T)/CBS accuracy and canonical variational transition state theory combined with small-curvature tunneling to study the reaction mechanism and kinetics of the hydrolysis of CH3CHO. The calculated results show that the hydrolysis of CH3CHO needs to overcome an energy barrier of 37.21 kcal mol−1, while the energy barrier is decreased to −9.79 kcal mol−1 with a sulfuric acid catalyst. In addition, the calculated kinetic results show that the H2SO4⋯H2O + CH3CHO reaction is faster than H2SO4 + CH3CHO⋯H2O. Additionally, the H2SO4⋯H2O + CH3CHO reaction can play an important role in the sink of CH3CHO below 260 K occurring during the night period when OH, H2SO4, and H2O concentrations are 104, 108, and 1017 molecules cm−3, respectively, because it can compete well with the CH3CHO + OH reaction. There are wide implications in atmospheric chemistry from these findings because of the potential importance of the catalytic effect of H2SO4 on the hydrolysis of CH3CHO in the atmosphere and in the formation of secondary organic aerosols.


 Please wait while we load your content...
Please wait while we load your content...